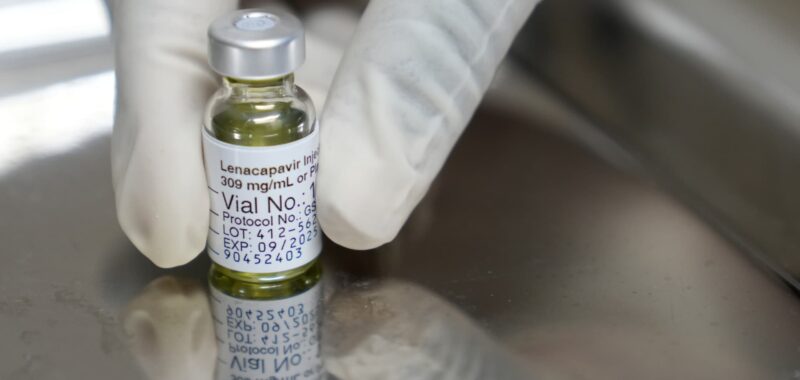
Gilead‘s twice-yearly shot reduced HIV infections by 96% in a second large study, the company said Thursday.
The positive phase three trial data on Lenacapavir sets the stage for likely approval by the U.S. Food and Drug Administration for HIV prevention.
Now that we have a comprehensive dataset across multiple study populations, Gilead will work urgently with regulatory, government, public health and community partners to ensure that, if approved, we can deliver twice-yearly lenacapavir for PrEP worldwide, for all those who want or need PrEP,” said Gilead CEO Daniel O’Day in a statement.
PrEP or, pre-exposure prophylaxis, is a medication taken to prevent getting HIV, according to the Centers for Disease Control and Prevention.
Gilead shares climbed about 3% in premarket trading Thursday.
The company said 99.9% of participants who received Lenacapavir did not acquire HIV, with two cases among 2,180 people. The trial included cisgender men, transgender men, transgender women and gender non-binary people who have sex with partners assigned male at birth.
Subscribe to CNBC on YouTube.