Evolution is quite a wondrous and lengthy process, with some random bursts of activity that are responsible for the diversity of life on our planet today. These can happen on large scales like with the evolution of more efficient limbs. They also occur at microscopic cellular level, such as when different parts of the cell were first formed.
Now, a team of scientists have detected a sign of a major life event that has likely not occurred for at least one billion years. They’ve observed primary endosymbiosis–two lifeforms merging into one organism. This incredibly rare event occurred between a type of abundant marine algae and a bacterium was observed in a lab setting. For perspective, plants first began to dot our planet the last time this happened. The results are described in two papers recently published in the journals Cell and Science.
Where the ‘powerhouse of the cell’ and chloroplasts come from
Primary endosymbiosis happens when one microbial organism engulfs another. It then begins to use the swallowed organisms as an internal organ. The host provides the organism–now called an endosymbiont–several benefits including nutrients, energy, and protection. When it can no longer survive on its own, the engulfed endosymbiont becomes an organ for the host called an organelle.
“It’s very rare that organelles arise from these types of things,” Tyler Coale, a co-author of the Cell study and a postdoctoral scholar at the University of California, Santa Cruz said in a statement. “The first time we think it happened, it gave rise to all complex life.”
Endosymbiosis where the host life form becomes fundamental to another organism’s function has only happened three known times. All of these instances were a major breakthroughs for evolution, since merging with their hosts became fundamental for the endosymbionts very existence.
The first event was roughly 2.2 billion years ago. This is when a single-celled organism called archaea swallowed up a bacterium that eventually became the mitochondria. This specialized organelle is what every biology student learns is the “powerhouse of the cell” and its formation allowed for complex organisms to evolve.
“Everything more complicated than a bacterial cell owes its existence to that event,” said Coale. “A billion years ago or so, it happened again with the chloroplast, and that gave us plants,” Coale said.
This second event occurred when more advanced cells absorbed cyanobacteria. Cyanobacteria can harvest energy from sunlight and they eventually become organelles called chloroplasts that can harvest energy from sunlight. The chloroplasts gave us another core principle of biology–green plants that can make food from the sun.
With this latest endosymbiosis event, it’s possible that the algae is converting nitrogen from the atmosphere into ammonia that it can use for other cellular processes. However, it needs the help of a bacterium.
A new organelle?
In the paper published in Cell, a team of scientists show that this process is occurring yet again. They looked at a species of algae called Braarudosphaera bigelowii. The algae engulfed a cyanobacterium gives it a bit of a plant superpower. It can “fix” nitrogen straight from the air and combine it with other elements to form more useful compounds. This is something that plants normally can’t do.
Nitrogen is a very important nutrient for life to exist and plants normally get it through mutual relationships with the bacteria that remain separate from the plant or algae. The team first thought that the B. bigelowii algae had this kind of symbiotic relationship with a bacterium called UCYN-A. The relationship had actually gotten much more close and serious.
[Related: You have no idea how much you need these bacteria.]
They found that the size ratio between the algae and UCYN-A bacterium remains similar across different species related to the B. bigelowii algae. The growth appears to be controlled by an exchange of key nutrients, linking up their metabolisms. This synchronization of growth rates led the researchers to call UCYN-A organelle-like.
“That’s exactly what happens with organelles,” study co-author and UC Santa Cruz microbial oceanographer Jonathan Zehr said in a statement. “If you look at the mitochondria and the chloroplast, it’s the same thing: they scale with the cell.”
Introducing the nitroplast
To look for more lines of evidence that this bactrium is an organelle, they needed to take a deeper look inside. The study published in the journal Science used advanced X-ray imaging to get a look at the interior of the living B. bigelowii algae cells. It revealed that the replication and cell division was synchronized between both the host algae and the UCYN-A bacterium. It provided even more evidence of this organism merging process of primary endosymbiosis at work.
“Until this paper, there was still a question of is this still an ‘endosymbiont’, or has it become a true organelle?” Carolyn Larabell, a study co-author and faculty scientist at Berkeley Lab’s Biosciences Area and Director of the National Center for X-Ray Tomography, said in a statement. “We showed with X-ray imaging that the process of replication and division of the algal host and endosymbiont is synchronized, which provided the first strong evidence.”
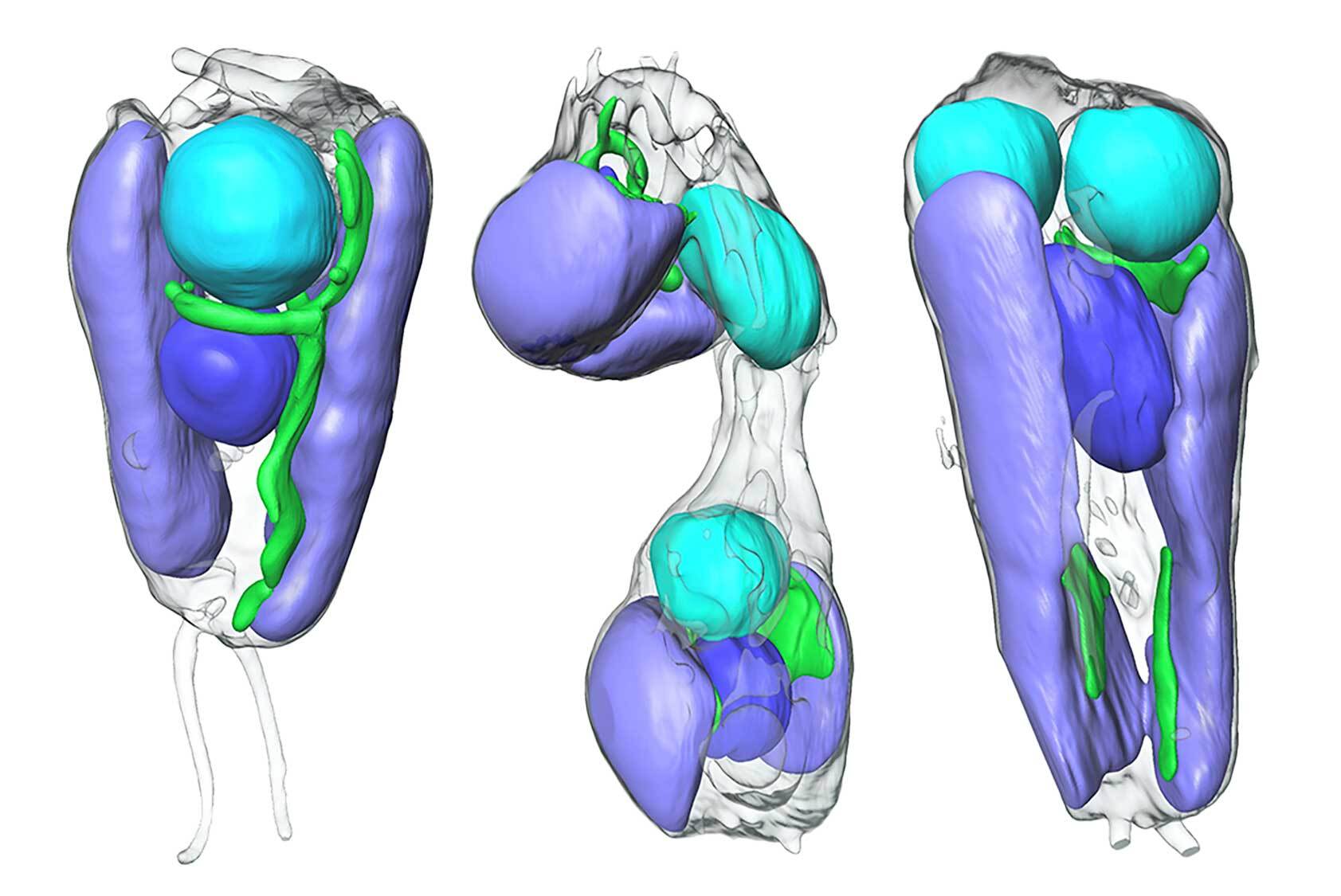
They also compared the proteins of isolated UCYN-A bacteria to the proteins inside of the algae cells. The team found that the isolated bacterium can only make roughly half of the proteins it needs. It needs its algal host to provide it with the rest of the proteins necessary for living.
“That’s one of the hallmarks of something moving from an endosymbiont to an organelle,” said Zehr. “They start throwing away pieces of DNA, and their genomes get smaller and smaller, and they start depending on the mother cell for those gene products–or the protein itself–to be transported into the cell.”
The team believes that this indicates that UCYN-A can be considered a full organelle. They gave it the name “nitroplast,” and it potentially began to evolve around 100 million years ago. While that sounds long to our human sense of time, it’s a mere millisecond in evolutionary time when compared with mitochondria and chloroplasts.
Plenty of other questions about UCYN-A and its algal host remain unanswered and the team also plans to figure out UCYN-A and the alga operate and study different strains. Further study of nitroplasts could also determine if they are present in other cells and what their benefits may be. For example, it could have wide applications in agriculture.
“This system is a new perspective on nitrogen fixation, and it might provide clues into how such an organelle could be engineered into crop plants,” said Coale.
According to Zehr, scientists will likely find other organisms that have similar evolutionary stories as UCYN-A, but this discovery is “one for the textbooks.”

