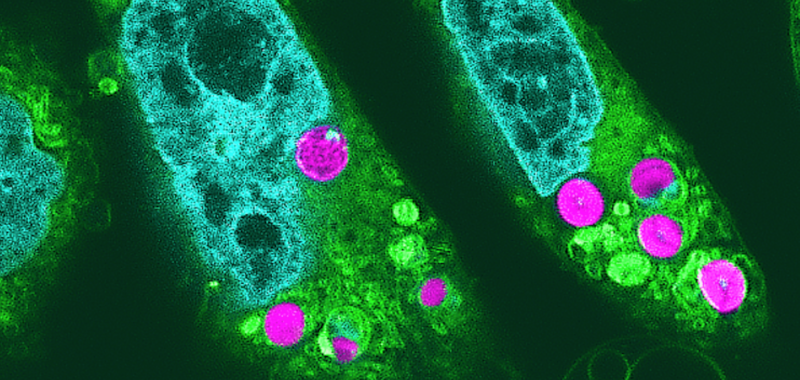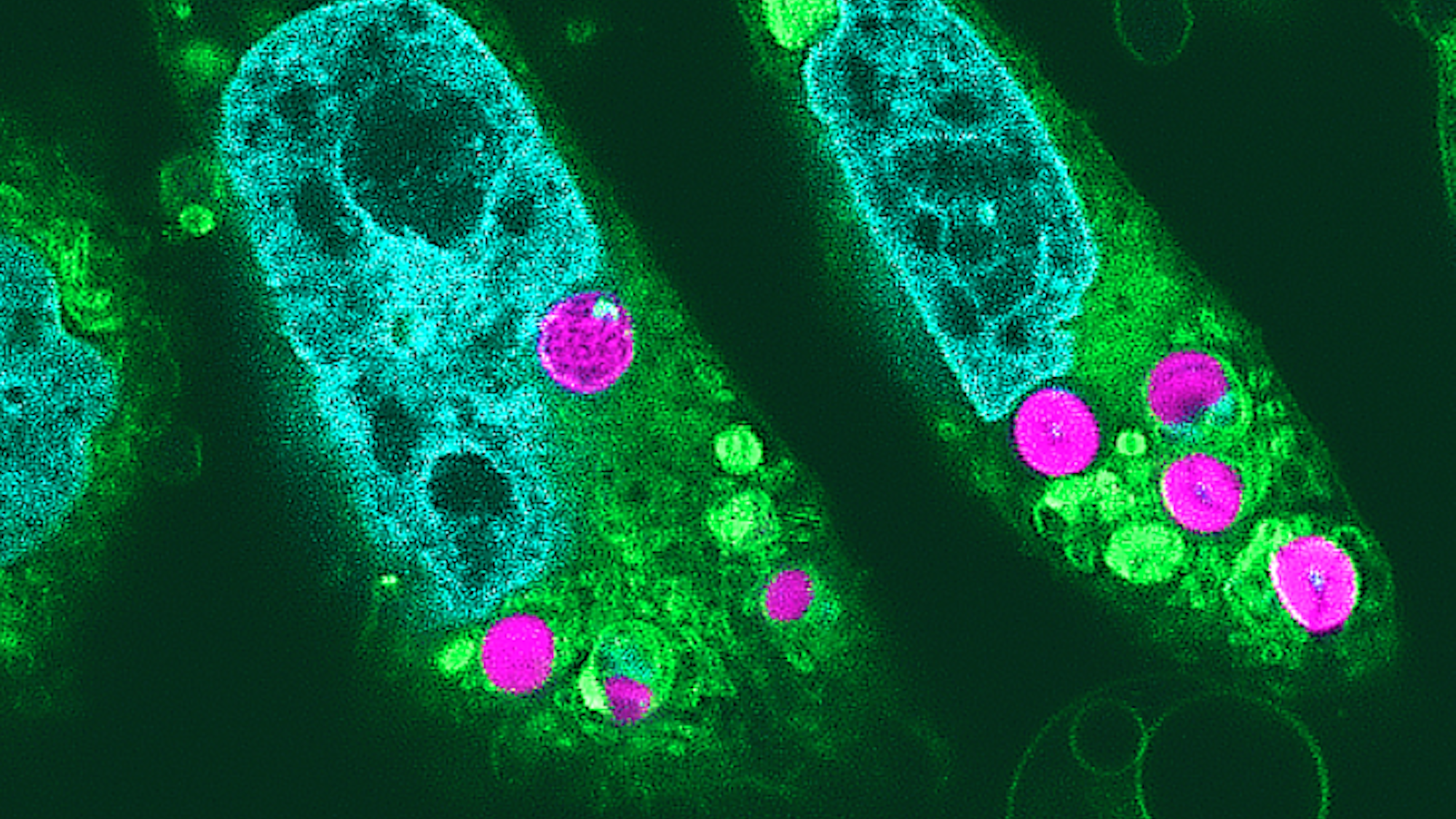
Researchers say they have successfully cultivated animal cells that draw energy through photosynthesis. Previously believed impossible, experts accomplished the first-of-its-kind feat thanks to two main ingredients: red algae, and hamster cells.
Plants use photosynthesis to derive much of their energy through cells filled with chlorophyll—called chloroplasts—that convert sunlight into life-sustaining power. Animals, on the other hand, aren’t naturally capable of generating chloroplasts. Most experts agreed it wasn’t possible to bioengineer hybrid cells to allow creatures to obtain energy or nutrients (other than vitamin D) from the sun. According to a study from a University of Tokyo team published in Proceedings of the Japan Academy, however, a future filled with “planimal” cells capable of photosynthesis may become reality.
“We thought that the chloroplasts would be digested by the animal cells within hours after being introduced,” paper corresponding author Sachihiro Matsunaga said in a statement last month. “However, what we found was that they continued to function for up to two days, and that the electron transport of photosynthetic activity occurred.”
Matsunaga’s team first harvested chloroplasts from red algae and inserted them into cultures of cells derived from hamsters. They then monitored the health and growth of cell structures using multiple imaging methods such as electron, confocal, and superresolution microscopy. Meanwhile, researchers utilized a strategy involving bursts of light called pulse amplitude modulation fluorometry to document and measure electron transport via photosynthetic activity. Where there was light, there was energy—meaning that the chloroplast-infused hamster cells benefited from photosynthesis.
“As far as we know, this is the first reported detection of photosynthetic electron transport in chloroplasts implanted in animal cells,” explained Matsunaga.
For up to two days, the modified animal cells displayed higher rates of cell growth, implying the new chloroplasts offered carbon-based fuel for their hosts. But while the results may inspire visions of one day getting much of your daily nutrition from simply sunbathing, for now, the implications are much more localized. But that doesn’t mean they are any less promising to researchers, as well as the wider medical community.
According to Matsunaga, lab-grown tissues like artificial organs, skin transplant sheets, and even artificial meat are all composed of multiple layers of cells. Often, however, cell cultures struggle to proliferate and survive due to hypoxia, or low oxygen levels. If scientists can reliably introduce chloroplasts into these cells, then additional oxygen could be derived through light irradiation-induced photosynthesis. This would hypothetically make lab-grown cell cultures easier, cheaper, and more eco-friendly to produce.
Researchers note in their study that previous experiments indicate these aren’t far-fetched goals. Scientists have already successfully modified metabolic paths in non-plant organisms such as E. coli bacteria and yeast to handle carbon fixation—the process of converting inorganic carbon into organic compounds for energy storage and other biological needs. If additional genomic modifications can be applied to cell cultures to sustain or even extend photosynthesis, then eventually “photosynthetic products [could be] compatible with host cell metabolism in mammalian cells,” the team writes. Matsunaga, for his part, feels confident in further advancements.
“We expect planimal cells to be game-changing cells, which in the future can help us achieve a ‘green transformation’ to a more carbon-neutral society,” he said.